PEEK Medical Research 3D Printing Filaments
Discover our certified medical research-grade PEEK filament, designed for high-performance 3D printing. PEEK pellets got ISO 10993, FDA-compliant, and rigorously tested for biocompatibility, sterilization, and mechanical excellence. Ideal for medical research 3d printing.
The difference between implantable-grade PEEK filament and medical research-grade PEEK 3D printing filament is that the implantable-grade filament have been certified to ISO 10993 and produced in an ISO 13485 compliant factory, and Research grade is only certified for peek particles.

Raw Materials Key Certifications & Properties:
USP Class VI / ISO 10993 Biocompatibility – Tested for safety in prolonged human implant contact.
FDA-Compliant – Formulated in accordance with FDA regulations for medical-grade polymers.
ISO 9001:2015 compliant factory.
Sterilization Resistance – Validated for autoclaving, gamma irradiation, and chemical sterilization.
Mechanical Excellence – High tensile strength, fatigue resistance, and stability under physiological conditions.

Why Choose Our Medical Research PEEK Filament?
Reliability: Ideal for researching implantable devices, surgical instruments, and custom prosthetics.
Precision: Consistent diameter and thermal properties ensure reliable printing outcomes.
Applications: All preliminary research on medical 3D printing (especially implant 3D printing).
Elevate your medical device manufacturing with a material trusted by leading healthcare innovators. Download our certification packages and technical data sheets to validate performance for your critical applications.
PEEK Medical Research 3D Printed Sample
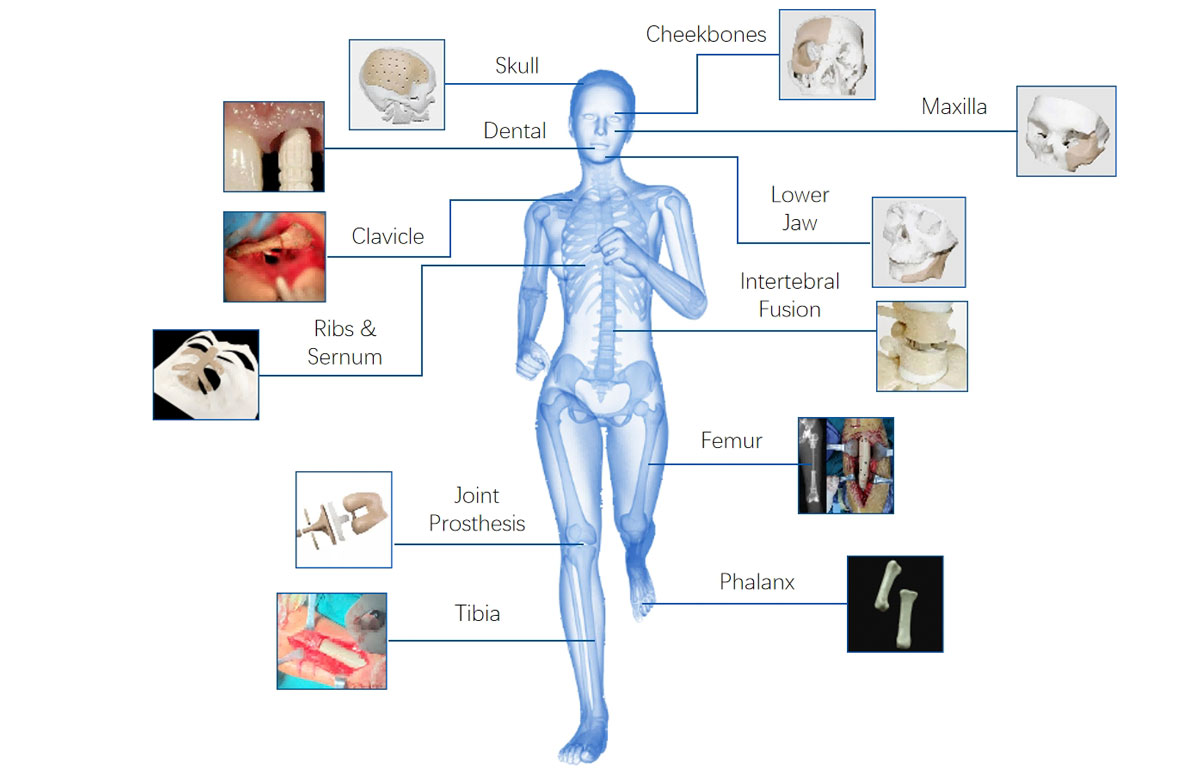


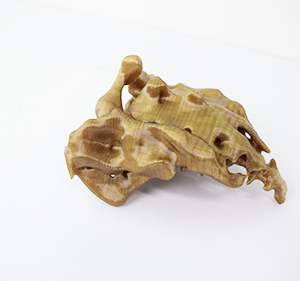

Applications Of PEEK Medical Research
Implantation Research in 3D Printing PEEK Material
PEEK Medical Research Certification
FDA Compliance

USP40 - NF35

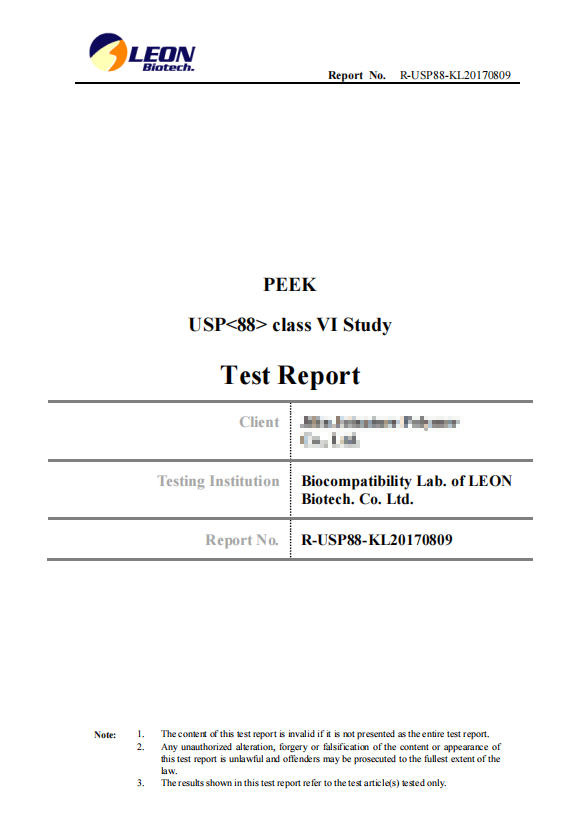
USP88 Class VI Study
ISO9001

Dermal Irritation / Corrosion Test

In Vitro Cytotoxicity - Qualitative Test of Agar Diffusion

In Vitro Cytotoxicity Test Elution Test

Skin Sensitization

